Chronic arterial occlusion
Adult: 100 mg tid, may be adjusted according to age and symptoms.
Elderly: May reduce to 150 mg daily.
Elderly: May reduce to 150 mg daily.
|
Indications and Dosage
Oral
Chronic arterial occlusion Adult: 100 mg tid, may be adjusted according to age and symptoms.
Elderly: May reduce to 150 mg daily. |
|
Contraindications
Haemorrhage, including gastrointestinal ulceration, urinary tract haemorrhage, haemophilia, capillary fragility, haemoptysis, vitreous haemorrhage. Pregnancy.
|
|
Special Precautions
Patient with haemorrhagic diathesis, bleeding tendency (e.g. gastrointestinal ulcer, haemoptysis, urinary tract haemorrhage, vitreous haemorrhage),or menstrual period. Severe renal impairment. Elderly. Lactation.
|
|
Adverse Reactions
Significant: Cerebral and gastrointestinal haemorrhage, thrombocytopenia, agranulocytosis, jaundice, increased AST/ALT and bilirubin.
Blood and lymphatic system disorders: Anaemia. Cardiac disorders: Palpitations, breath shortness, chest pain. Gastrointestinal disorders: Abdominal pain, nausea, heartburn, constipation, anorexia, diarrhoea. General disorders and administration site conditions: Malaise. Investigations: Increased blood triglycerides and cholesterol, decreased serum albumin and serum calcium, increased weight. Metabolism and nutrition disorders: Oedema. Nervous system disorders: Headache, dizziness, taste abnormality. Psychiatric disorders: Sleepiness. Renal and urinary disorders: Proteinuria, increased BUN and creatinine, urinary sugar, abnormal urinary sediment. Respiratory, thoracic and mediastinal disorders: Epistaxis. Skin and subcutaneous tissue disorders: Rash, redness, pruritus. Vascular disorders: Hot flush. |
|
Monitoring Parameters
Monitor for abnormal haemorrhage and LFT.
|
|
Drug Interactions
Increased risk of bleeding with anticoagulants (e.g. warfarin) and antiplatelets (e.g. cilostazol, aspirin, ticlodipine).
|
|
Action
Description:
Mechanism of Action: Sarpogrelate hydrochloride inhibits platelet aggregation and vascular contraction through antagonistic action to 5-HT2 serotonin receptor in platelets and vascular smooth muscle thereby preventing blood clot formation and improving blood flow. Pharmacokinetics: Metabolism: Deesterified and further metabolised by multiple CYP enzymes (CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP3A4). Excretion: Via urine (44.5%) and faeces (4.2%). |
|
Chemical Structure
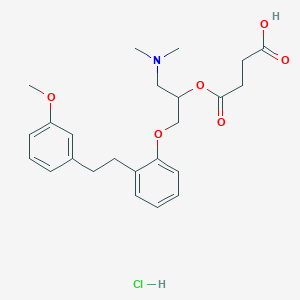 Source: National Center for Biotechnology Information. PubChem Database. Sarpogrelate hydrochloride, CID=444005, https://pubchem.ncbi.nlm.nih.gov/compound/Sarpogrelate-hydrochloride (accessed on Jan. 23, 2020) |
|
Storage
Store between 20-25°C. Protect from light and moisture.
|
|
References
Buckingham R (ed). Sarpogrelate Hydrochloride. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 08/05/2018 .
|